A
Grade
Carboplatin
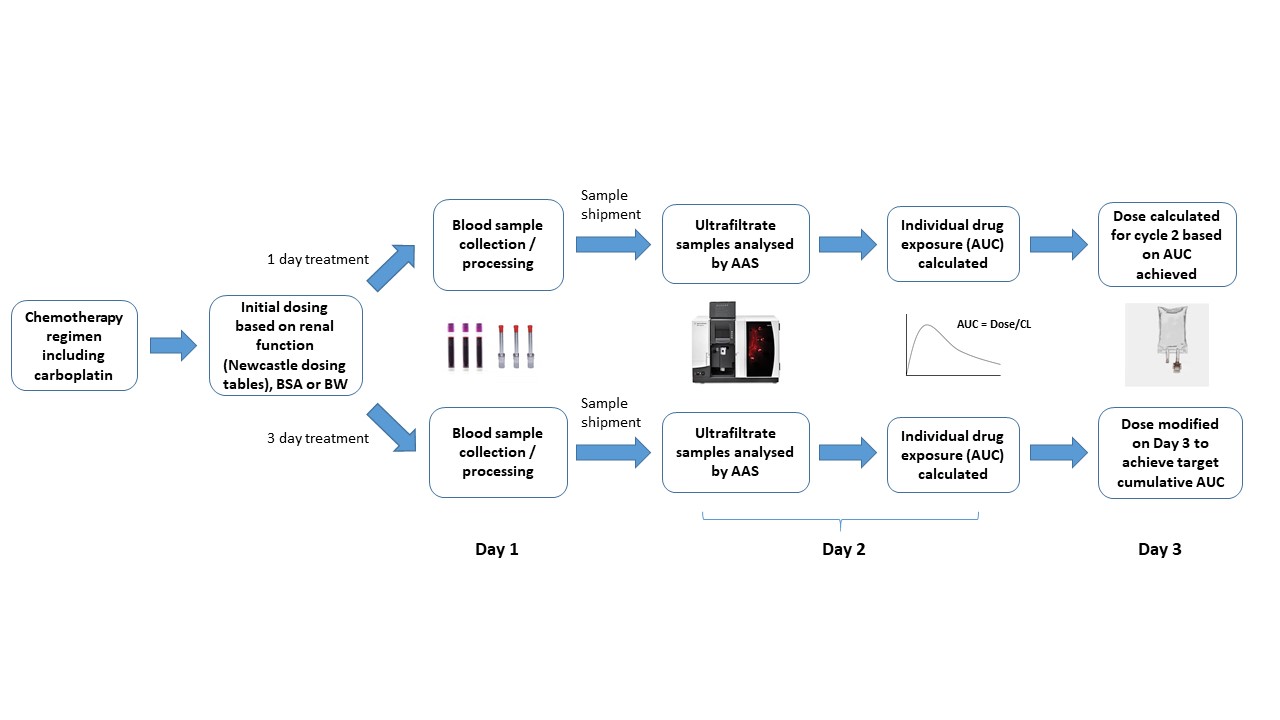
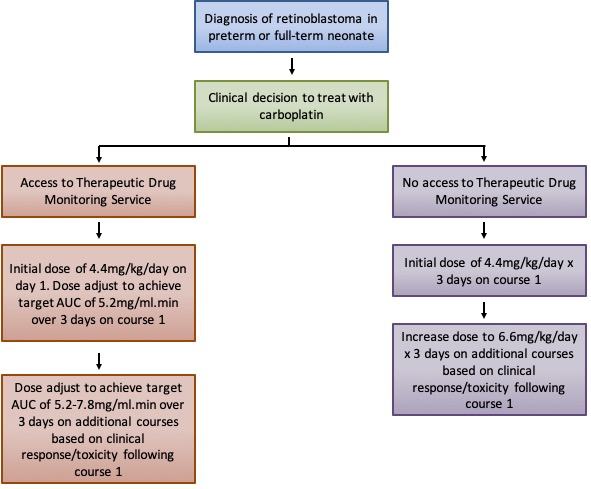
Carboplatin represents the "gold standard" of TDM in paediatric oncology (Figure 1). There is a clear understanding of the relationship between free drug exposure and toxicity/response in adult and paediatric patients. For the youngest of patients (<10kg), a mg/kg based dosing approach alongside TDM is advised (Figure 2). For cases where TDM is not feasible, starting at a lower dose of 4.4mg/kg on the first cycle and increasing to 6.6mg/kg on subsequent cycles if the dose is well tolerated is advised (Figure 2).
Level 1
Population PK model including infants
| Author | Method | Number of Patients/Infants(<1yrs) | Age(yr), Median(range) | Age related findings | ||
|---|---|---|---|---|---|---|
| Chatelut (1996) | Two compartment popPK model (free platinum). CL corrected for BW, creatinine and nephrectomy | 57 / NS | 5 (0.17-18) | No additional effect of age on PK parameters that was not explained by BW or BSA. | ||
| Tonda (1996) | One compartment popPK model (free platinum) | 21 / 4 | 1.7 (0.2-4.2) | Median CL (mL/min/m2) in infants lower than median for older patients. | ||
| Thomas (2000) | Two compartment popPK model (free platinum) | 38 / NS | 3.5 (0.4-16.3) | No effect of age on dosing method (renal function vs BSA dosing). | ||
| Patoux (2001) | Two compartment popPK model (free platinum). Serum creatinine was identified as the most significant covariate on CL. | 117 / NS | 6 (0.1-18.4) | No effect of age on CL relative to other covariates tested. | ||
| Veal (2010) | Two compartment popPK model (free platinum). BW was included as covariate on PK parameters. | 19 / 16 | 0.8 (0.2-1.2) | CL (mL/min/kg) higher in infants <12kg compared to children >12kg. | ||
| Veal (2015) | Therapeutic drug monitoring in preterm and full-term neonates. CL and AUC were calculated using the PK model of Veal (2007) and Peng (1995). | 9 / 11 | 3 weeks (0.4-24 weeks) (gest. age 40 weeks (35-52 weeks)) | CL (mL/min and mL/min/kg) increased with increasing age (normalized to full-term gestation). |
Level 2
PK model including infants or PopPK model without infants
| Author | Method | Number of Patients/Infants(<1yrs) | Age(yr), Median(range) | Age related findings | ||
|---|---|---|---|---|---|---|
| Madden (1992) | Two compartment popPK model (total and free platinum) | 18 / 0 | 7.7 (2.9-23) | No effect of age on CL after normalization to BW | ||
| Peng (1995) | Two compartment popPK model (total and free platinum) | 23 / 3 | 3.7 (0.08-18.4) | Effect of age was not studied. | ||
| Doz (1998) | Two compartment popPK model (free platinum) | 15 / 1 16 / 1 | 5.0 (0.9-10.7) 4.6 (0.75-17.5) | Effect of age was not studied. | ||
| Rubie (2003) | Two compartment popPK model (free platinum) | 12 / 0 | 3.4 (2.8-17.3) | Effect of age was not studied. | ||
| Veal (2007) | Two compartment popPK model (free platinum) | 28 / 0 | 11.7 (1-21) | Effect of age on PK was not studied. No age effect on platinum adduct formation. | ||
| Duong (2019) | Two compartment popPK model (free platinum). Allometric scaling for BW. | 46 / 0 | 3.5 (1.7-8.3) | No effect of age on the PK parameters after including BW into the model. | ||
| Hong (2020) | Non-compartmental pharmacokinetics and one compartment popPK model (total platinum). eGFR was included as covariate on CL. | 25 / 0 | 6 (1-17) | Effect of age was not studied. |
Level 3
Non-compartmental PK study or PK model without infants
| Author | Method | Number of Patients/Infants(<1yrs) | Age(yr), Median(range) | Age related findings | ||
|---|---|---|---|---|---|---|
| Newell (1993) | Two compartment PK model (total and free platinum) | 22 / 4 | 3.8 (0.3-15.8) | Effect of age was not studied only the impact of EDTA CL on dosing. Significant correlation between EDTA CL and carboplatin CL. | ||
| Riccardi (1994) | Two compartment PK model (free platinum) | 35 / NS | 7 (0.8-17) | Effect of age was not studied but PK parameters comparable to those reported in adults. | ||
| Kangarloo (2004) | Non-compartmental pharmacokinetics and PK model (total and free platinum) | 10 / 2 | 11 (0.25-16) | Effect of age was not studied. | ||
| Levy (2009) | Non-compartmental pharmacokinetics (free platinum) | 28 / 0 | 8.5 (1-21) | Effect of age was not studied. |
Level 4
Case-series/report
| Author | Method | Number of Patients/Infants(<1yrs) | Age(yr), Median(range) | Age related findings | ||
|---|---|---|---|---|---|---|
| Riccardi (1992) | Case series, two compartment PK model (total and free platinum) | 5 / 0 | 6 (1.5-7) | Effect of age was not studied. | ||
| Picton (2009) | Case series, two compartment PK model (free platinum) | 1 / 1 | Gest. age 32 weeks | CL (mL/min) increased by 2-fold over 7 weeks. | ||
| Veal (2016) | Case series, non-compartmental pharmacokinetics (free platinum) | 1 / 1 | 8 weeks (gest. age 40 weeks) | CL normalized for BW comparable with reported values. | ||
| Hawley (2019) | Case series, non-compartmental pharmacokinetics (free platinum) | 1 / 1 | 5 weeks (gest. age 40 weeks) | Effect of age not discussed, but under standard dosing regimen patient would have been under-dosed |
Level 5
Mechanism-based reasoning or expert opinion
| Author | Method | Number of Patients/Infants(<1yrs) | Age(yr), Median(range) | Age related findings | ||
|---|---|---|---|---|---|---|
| Balis (2017) | Mechanism-based development of dose bands based on BSA intervals |