A
Grade
Vincristine
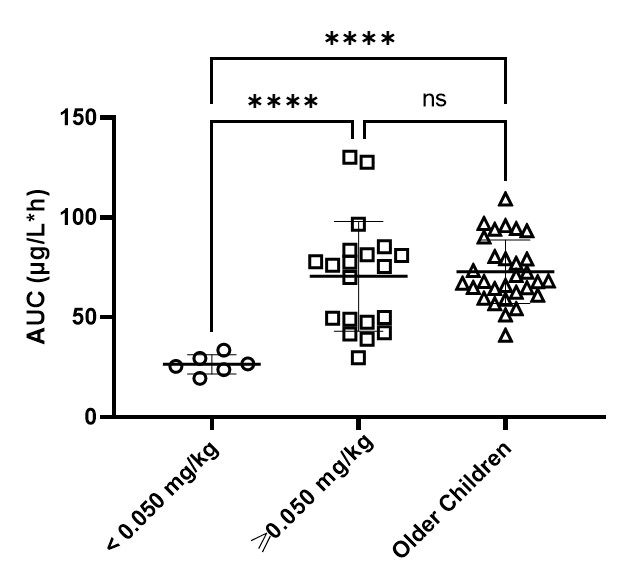
TDM of vincristine is recommended in infants and paediatric patients with hepatic impairment or excessive toxicity. A target window for vincristine exposure of 50-100 µg/L*h has recently been identified. Dosing infants at 0.05mg/kg or greater resulted in drug exposures comparable to older children and was generally well tolerated (Figure 3). However, for infants dosed at <0.05mg /kg, significantly lower exposures relative to older children and infants dosed at ≥0.05mg/kg have been observed (Figure 3). Therefore, it is advised to start vincristine dosing at a dose level of at least 0.05mg/kg and guide dosing using TDM.
Level 1
Population PK model including infants
| Author | Method | Number of Patients/Infants(<1yrs) | Age(yr), Median(range) | Age related findings | ||
|---|---|---|---|---|---|---|
| Barnett (2021) | Two compartment popPK model. Allometric scaling for BW. Age was included as covariate on V2 | 57 / 21 | 5.6 (0.04-17.2) | No significant difference in BSA-normalized CL between infants and older children. There was a trend towards lower CL in neonates (0-4 weeks) as compared to infants (1-12 months). Doses of <0.05mg/kg result in significantly lower AUC values than observed in neonates and infants receiving doses of ≥0.05mg/kg, and in older children receiving a dose of 1.5mg/m2. |
Level 2
PK model including infants or PopPK model without infants
| Author | Method | Number of Patients/Infants(<1yrs) | Age(yr), Median(range) | Age related findings | ||
|---|---|---|---|---|---|---|
| Crom (1994) | Two compartment PK model | 54 / 2 | 4.3 (0.2-18) | No correlation between CL (mL/min/m2) and age. A significant correlation between CL (mL/min/kg) and age. CL in children in higher than in adults, but CL in two infants was lower. | ||
| Gidding (1999) | Two compartment PK model | 32 / NS | 4.6 (0-16) | CL (mL/min/m2) in infants under 1 year is lower than in older children. | ||
| Guilhaumou (2011) | Two compartment popPK model | 26 / 0 | NS (2.0-16.0) | No effect of age on the PK parameters. | ||
| Moore (2011) | One compartment popPK model. Allometric scaling for BW. | 50 / 0 | 6.5 (1.0-16.25) | No effect of age on the PK parameters. | ||
| Van de Velde (2020) | Two compartment popPK model. PK parameters were normalized to BSA. Duration of infusion was included as covariate on Q and V2. | 35 / 0 | 10.06 (NS) | Effect of age was not described. |
Level 3
Non-compartmental PK study or PK model without infants
| Author | Method | Number of Patients/Infants(<1yrs) | Age(yr), Median(range) | Age related findings | ||
|---|---|---|---|---|---|---|
| De Graaf (1995) | Two compartment PK model | 17 / 0 | 3.8 (1.3-12.4) | CL (mL/min/m2) in children appeared to be more than twice as large as in adults and t1/2 in adults was much longer than in children. | ||
| Groninger (2002) | Two compartment PK model | 70 / 0 | NS (1-16) | No effect of age on CL (mL/min/m2). | ||
| Frost (2003) | Two compartment PK model | 98 / 0 | 4.5 (1.3-17.3) | No effect of age on the PK parameters. | ||
| Plasschaert (2004) | Two compartment PK model | 52 / 0 | NS (1-16) | No effect of age on CL (mL/min/m2). | ||
| Groninger (2005) | Two compartment PK model | 54 / 0 | NS (1-16) | Effect of age was not studied. | ||
| Lönnerholm (2008) | Two compartment PK model (with data of Frost 2003) | 86 / 0 | NS (1.2-17.4) | No effect of age on the PK parameters. | ||
| Skolnik (2021) | Non-compartmental pharmacokinetics | 132 / 9 | NS (0.21-16.8) | Age had a minimal effect on variability of PK. Compared to children <1 year, the BSA-adjusted dose was 44-79% higher older children. Consistent with the differences in dose, the median AUC was lowest for children <1 year and highest in older children. CL did not appear to be related to age. |
Level 5
Mechanism-based reasoning or expert opinion
| Author | Method | Number of Patients/Infants(<1yrs) | Age(yr), Median(range) | Age related findings | ||
|---|---|---|---|---|---|---|
| Balis (2017) | Mechanism-based development of dose bands based on BSA intervals | |||||
| Lee (2019) | Physiological-based PK model. Intracellular binding to β-tubulin was included as covariate. | 25 / NS | NS (0.4-9) | Simulating a higher hypothetical (4.9-fold) pediatric expression of β-tubulin relative to adult improved predictions PK. |